Document Type : Review Article
Authors
1 Department of Chemical Sciences, Federal University PMB, 1020, Wukari
2 Department of Chemistry, Rivers State University, Nkpolu-Oroworukwo, Port Harcourt
Abstract
Supercritical fluids have emerged as a unique and promising class of materials for various applications owing to their tunable physical and chemical properties. These fluids are characterized by their state above the critical temperature and pressure, where they exhibit properties of both liquid and gas phases. Their properties, including density, viscosity, and diffusivity, can be finely tuned by precisely controlling their pressure and temperature. Furthermore, they possess excellent solvating properties that can dissolve a wide range of organic and inorganic compounds. Supercritical fluids are utilized in diverse fields, including materials science, pharmaceuticals, energy, and environmental remediation. Some of the popular applications include supercritical fluid extraction, supercritical drying, supercritical fluid chromatography, and supercritical fluid in materials sciences. This review provides an overview of the basic principles of supercritical fluids, their unique properties, and their recent advances and applications in different scientific fields
Keywords
Main Subjects
Introduction
Substances that are above their critical point and display characteristics of both liquids and gases are known as supercritical fluids. The temperature and pressure range at which discrete liquid and gas phases of a substance vanish and it transforms into a homogenous fluid with special characteristics is known as the critical point. The critical point is represented on a phase diagram by a critical temperature (Tc) and critical pressure (Pc). A supercritical fluid is a substance that is at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can diffuse through solids like a gas, and dissolve materials like a liquid [1].
Above Tc and Pc the material is in a single homogeneous state with properties of those between that of liquid and gas. The density of a liquid increases with temperature whereas that of a gas increases with pressure; at a critical point, both become equal. SCFs typically have diffusivities closer to gases and densities closer to liquids, which results in high diffusion rates. Temperature and pressure changes can modify the SCFs properties as long as they stay above the critical point. Fluids such as supercritical xenon, ethane and carbon dioxide have a range of unusual chemical possibilities [2]. Supercritical fluids are of interest primarily because of their easily tunable properties, which may be altered by monitoring temperature and pressure. From very low solvent power at low densities (temperature near or higher than critical temperature and pressure lower than critical pressure) to good solvent power at high densities (temperature near or higher crucial temperature and pressure far over critical pressure) [3].
Due to regulatory and environmental demands on hydrocarbon and ozone-depleting emissions, organic solvents used in industrial purification and recrystallization operations are being replaced with supercritical fluids (SCFs). The SCFs usage is expanding quickly across all industrial sectors due to increased scrutiny of solvent residues in medicines and medical products. A substance enters the supercritical fluid state when its temperature and pressure exceed their critical values [4].
The substance does not show a significant separation between the liquid and gas phases when it is in this condition. Rather, it exhibits a spectrum of qualities, combining the traits of the two stages. This results in the formation of various unique characteristics of supercritical fluids, including viscosity and diffusivity, density and compressibility, solvating power, and transport properties [5].
The aim of the article is to explore supercritical fluids, its principle, formation, and applications. The article seeks to explore the applications of supercritical fluids in industries and their process of formations.
Phase system
Transition from gas to liquid state
The transition from the gas phase to the liquid phase typically occurs when a substance is subjected to increasing pressure while maintaining a constant temperature. As pressure increases, the molecules of the gas become closer together, and intermolecular forces start to play a more significant role. At a certain pressure, known as the vapour pressure, the gas phase condenses into a liquid phase [6].
Nevertheless, the substance experiences a crucial change in behaviour when the pressure rises further. The material transforms into a supercritical fluid at the critical point, where the boundary between the gas and liquid phases vanishes. It is possible for the liquid and gas phases to coexist at temperatures and pressures higher than the critical point. The supercritical fluid's density rises and its compressibility falls at pressures and temperatures over the critical point. Changes in the substance's thermodynamic properties coincide with this transition. In the supercritical area, for instance, there are notable changes in the heat capacity, thermal conductivity, and diffusion coefficients [7].
There are distinct benefits in a number of applications when transitioning from the gas phase to the supercritical fluid state [8]. By changing the pressure and temperature, supercritical fluids offer a customizable solvent system with variable characteristics. Exact control over the processes of extraction, separation, and reaction is made possible by this flexibility.
All things considered, the boundary that unlocks the extraordinary qualities and uses of supercritical fluids is the shift from the gas phase to the supercritical fluid state [9].
Properties of supercritical fluids
Density and compressibility
Density and compressibility are important properties that distinguish supercritical fluids from gases and liquids. In the supercritical region, the density of the fluid is comparable to that of a liquid, while its compressibility is closer to that of a gas. Supercritical fluids exhibit densities that are higher than those of gases but lower than those of liquids. The density of a supercritical fluid can be adjusted by varying the pressure and temperature conditions [10].
As pressure increases, the intermolecular distances decrease, leading to an increase in density. The density of a supercritical fluid can be calculated using equations of state, such as
 Figure 1: Carbon dioxide pressure-temperature phase diagram [9].
Figure 1: Carbon dioxide pressure-temperature phase diagram [9].
the Peng-Robinson equation or the Redlich-Kwong equation. These equations take into account factors like temperature, pressure, and molecular interactions to estimate the density of the fluid. For example, in supercritical carbon dioxide (CO2), the density ranges from approximately 0.3 g/cm³ to 1 g/cm³, depending on the temperature and pressure conditions. This density tunability makes supercritical fluids versatile for various applications, such as extraction and separation processes [11].
Compressibility refers to the change in volume of a substance with respect to changes in pressure. Supercritical fluids have a higher compressibility compared to liquids but lower compressibility compared to gases. This means that they are more compressible than liquids but less compressible than gases. The compressibility factor (Z) is often used to describe the compressibility of supercritical fluids. The compressibility factor provides information about the fluid deviation from ideal gas behaviour. Supercritical fluids generally exhibit compressibility factors close to unity. However, at higher pressures and temperatures, deviations from ideal behaviour can occur. These deviations can be attributed to molecular interactions and the presence of intermolecular forces [12].
Viscosity and diffusivity
Important supercritical fluid transport characteristics that affect mass transfer and fluid flow behaviour include viscosity and diffusivity. A fluid's resistance to flow is measured by its viscosity. Generally speaking, supercritical fluids have viscosities that are lower than those of liquids, which allow them to be highly mobile and effective for mass transit. Because there are fewer intermolecular forces and more molecules moving about in the supercritical state, there is less viscosity. Temperature, pressure, and the fluid's composition can all have an impact on a supercritical fluid's viscosity. Because of the greater molecular mobility at higher temperatures, viscosity often decreases [13].
conversely, as pressure increases, the viscosity may increase due to greater molecular interactions. Several empirical and semi-empirical models exist to estimate the viscosity of supercritical fluids, such as the Lucas-Washburn equation or the Wilke-Chang equation. These models take into account factors like temperature, pressure, and molecular size to predict the viscosity of the fluid. For instance, supercritical water (SCW) exhibits a relatively low viscosity compared to ambient water. At the critical point (374 °C, 221 bar), the viscosity of SCW is approximately 0.3 cP (centipoise), whereas at room temperature and pressure, water viscosity is around 1 cP [14].
The lower viscosity of SCW enhances its mass transfer properties, making it suitable for various chemical and biological applications. The rate at which molecules or particles spread or diffuse through a material is referred to as diffusivity. Diffusion is more akin to a gas in the supercritical state than a liquid, facilitating effective solute mixing and transport. A diffusivity of the supercritical fluid is influenced by its solute's composition, temperature, and pressure [15].
In general, diffusivity rises with temperature due to an increase in molecular mobility. Similarly, because there are fewer molecular interactions at lower pressures, diffusivity can be improved. The diffusivity of solutes in supercritical fluids can be estimated using empirical models, such as the Wilke-Chang equation or the Hayduk-Laudie equation. These models take into account factors like temperature, pressure, molecular size, and solute-solvent interactions to predict diffusivity. For example, in supercritical CO2, the diffusivity of solutes can range from 10^-5 to 10^-6 cm²/s. This high diffusivity allows for rapid extraction and efficient separation processes, making supercritical CO2 extraction a popular technique in various industries [16].
Solubility
In comparison to their solubility in liquids or gases, solutes' solubility in supercritical fluids can differ dramatically. When compared to conventional organic solvents, supercritical fluids frequently show better solubility for non-polar molecules. How soluble a solute is can be affected by a number of variables in supercritical fluids, such as pressure, temperature, solute type, and supercritical fluid nature [17].
Because the fluid has a higher density at higher pressures, solubility usually increases. Solubility is influenced by temperature as well; greater temperatures are frequently associated with higher solubility. Supercritical carbon dioxide (CO2) is one of the most widely used supercritical fluids. It has excellent solvating power for non-polar and lipophilic compounds, such as essential oils, flavours, and natural products. On the other hand, polar compounds, such as sugars and certain organic acids, exhibit lower solubility in supercritical CO2 unless co-solvents are employed [18].
Selectivity
Certain molecules can be extracted and separated from complex mixtures relative to the selective solvating power that supercritical fluids can display. The varied solubility of different solutes in the supercritical fluid is the cause of this selectivity. Target compounds can be extracted or separated with selectivity while undesirable components are left behind by varying the supercritical fluid's composition, temperature, and pressure. In applications where maintaining the integrity of bioactive substances is essential, such natural product extraction, this selectivity is extremely important [19].
Transport properties
Supercritical fluids are very effective for various transport processes, such as reaction kinetics, extraction, and separation, given their excellent mass transfer characteristics [20].
Mass transfer
Supercritical fluids are considered to have higher diffusivity and lower viscosity than liquids, which contributes to their high mass transfer rates. The solutes can move through the fluid quickly and effectively thanks to these characteristics. Because of their advantageous characteristics, supercritical fluids have improved mass transfer capacities. For instance, supercritical CO2 has low viscosity and high diffusivity, allowing for efficient extraction of compounds from solid matrices. The mass transfer coefficient (K) is commonly used to characterize the mass transfer rate in supercritical fluid systems. It is influenced by factors such as pressure, temperature, fluid velocity, and the nature of the solute and matrix [21].
Formation of supercritical fluids
The formation of supercritical fluids is primarily determined by the pressure and temperature conditions applied to a substance. By manipulating these parameters, it is possible to induce the transition from the gas or liquid phase to the supercritical state.
Pressure
A supercritical fluid is formed primarily by increasing pressure. The intermolecular forces between a substance's particles get stronger as pressure increases. As a result, the interparticle distances decreases and the density increases [22]. A substance can exist in a supercritical state above a certain pressure value, known as the critical pressure (Pc). Lower than the critical pressure, the material stays in the gaseous or liquid phase. Still, the material becomes a supercritical fluid when the critical pressure is exceeded. For example, the critical pressure of carbon dioxide (CO2) is approximately 73.8 bar (1071 psi). When CO2 is subjected to pressures above this value, it becomes a supercritical fluid [23].
Temperature
Another important factor in the formation of supercritical fluids is temperature. Increasing the temperature gives the particles more energy, which improves their mobility and weakens the interactions between molecules. The precise temperature at which a substance can exist in its supercritical state is known as the critical temperature (Tc). The material does not change from the gas or liquid phase at temperatures below the critical temperature. However, if the material is heated above the critical point, it becomes a supercritical fluid. For instance, the critical temperature of CO2 is approximately 31.1 °C (88°F) [24]. When CO2 is exposed to temperatures above this value, it enters the supercritical state. The pressure and temperature conditions required to form a supercritical fluid can be visualized using a phase diagram. The phase diagram illustrates the boundaries between the different phases (solid, liquid, gas, and supercritical) as a function of pressure and temperature [25].
Supercritical fluid Applications
Supercritical fluid extraction
Supercritical fluid extraction (SFE) is one of the most prominent applications of supercritical fluids. It is a separation technique used to extract desired components from solid or liquid matrices using supercritical fluids as solvents. SFE offers several advantages over conventional extraction techniques, making it a preferred choice in various industries [6]. Supercritical fluid extraction operates on the principle that supercritical fluids exhibit unique solvent properties, including tunable solvating power, high diffusivity, and low viscosity. These properties enable efficient extraction of target components from complex matrices. The SFE process involves the following steps:
- Selection of Supercritical Fluid: The choice of supercritical fluid depends on the desired application and the solubility characteristics of the target components. Carbon dioxide (CO2) is the most commonly used supercritical fluid due to its favorable properties, such as low toxicity, non-flammability, and ease of handling [17].
- System Setup: The high-pressure vessel, known as an extractor, that has temperature and pressure controls is the main component of the extraction system. Placed inside the extractor is the liquid or solid matrix that contains the target components.
- Pressurization: The system is pressurized to reach the desired pressure above the critical point of the selected supercritical fluid. The pressure is typically in the range of 100 to 400 bar (1450 to 5800 psi), depending on the solubility requirements.
- Heating: The system is heated to reach the desired temperature above the critical temperature of the supercritical fluid. The temperature is typically in the range of 35 to 60 °C (95 to 140 °F) for CO2 [15].
- Extraction: As the supercritical fluid passes through the matrix, it solubilizes the target components. The soluble components diffuse into the supercritical fluid, resulting in their extraction from the matrix.
- Separation: The recovered components are then removed from the supercritical fluid and returned to the gas phase by depressurizing or cooling the fluid. Once removed, the matrix material is removed and the extracted components are recovered [9].
Advantages over Conventional Extraction Techniques: Supercritical fluid extraction offers several advantages over conventional extraction techniques, including:
- Selectivity: Target components can be extracted selectively from supercritical fluids while undesirable compounds are left behind due to their tunable solvating power. Selectivity can be maximized by modifying the supercritical fluid's composition, pressure, and temperature.
- High Efficiency: Supercritical fluid extraction operates at lower viscosities and higher diffusivities compared to liquid-based extractions. This facilitates faster mass transfer, resulting in higher extraction efficiencies and reduced extraction times [3].
- Mild Operating Conditions: Because supercritical fluid extraction is often carried out at low pressures and temperatures, it helps protect the integrity of substances that are thermally sensitive. Because it reduces the degradation or loss of volatile chemicals, this is especially advantageous for the extraction of natural goods, essential oils, tastes, and fragrances [26].
- Environmentally Friendly: Supercritical fluid extraction often employs carbon dioxide as the solvent, which is non-toxic, non-flammable, and readily available. The use of supercritical CO2 as a solvent eliminates the need for organic solvents, reducing environmental pollution and minimizing health hazards [1]. Examples include extraction of natural products, essential oils, flavours, and fragrances [17].
Supercritical fluid chromatography
Supercritical Fluid Chromatography (SFC) is a form of normal chromatography, which is used for the analysis and purification of low to moderate molecular weight, thermally labile molecules. It can also be used for the separation of chiral compounds. Principles are similar to those of high performance liquid chromatography (HPLC); however, SFC typically utilizes carbon dioxide as the mobile phase; therefore, the entire chromatographic flow path should be pressurized. SFC is now commonly used for chiral separations and purifications in the pharmaceutical industries. SFC with CO2 utilizes carbon dioxide pumps that require that the incoming CO2 and pump heads be kept cold in order to maintain the carbon dioxide at a temperature and pressure that keeps it in a liquid state where it can be effectively used at some specified flow rate [18].
The CO2 becomes supercritical post the injector and in the column oven when the temperature and pressure are subjected to rise above the triple point of the liquid and the supercritical state is achieved. SFC has been compared to a chromatographic process that combines the kinetics and chromatographic interactions of a gas with the ability of a liquid to dissolve a matrix. As a result, you can retain a high chromatographic efficiency while getting a lot of mass on each column per injection [5]. Through the use of an automatic back pressure regulator, SFC offers pressure as an additional control parameter. Because just the analyte and a tiny amount of polar co-solvent remain after the primary mobile phase evaporates, fraction collection in SFC is more practical from an operational standpoint even though it is just as straightforward and reliable as HPLC [19].
If the outlet CO2 is captured, it can be recompressed and recycled, allowing for >90% reuse of CO2. Any molecule that will dissolve in methanol or a less polar solvent is compatible with SFC, including polar solutes. The mobile phase is composed primarily of supercritical carbon dioxide, but since CO2 on its own is too non-polar to effectively elute many analytes, co-solvents are added to modify the mobile phase polarity. Co solvents are typically simple alcohols like methanol, ethanol, or isopropyl alcohol [17]. Supercritical fluid chromatography operates based on the principles of adsorption and partition.
Supercritical Fluids in Energy and Fuels
Supercritical fluids have significant applications in the energy and fuels sector. Their unique properties and tunable characteristics make them valuable in various processes, including the extraction and purification of bio fuels, carbon capture and storage, and enhanced oil recovery. Supercritical fluids offer advantages such as high solubility, selective extraction, and efficient mass transfer, making them effective tools in energy-related applications.
- Extraction and Purification of Bio-fuels: Supercritical fluids, particularly supercritical carbon dioxide (scCO2), play a crucial role in the extraction and purification of bio-fuels from renewable sources. Bio-fuels, such as biodiesel and bioethanol, can be obtained from biomass feedstock through extraction processes using supercritical fluids. Compared to the conventional solvents, supercritical CO2 has several benefits, such as excellent selectivity, low toxicity, and mild operation. It enables the effective extraction of contaminants while maintaining the extraction of bio-fuel components, such as sugars from lignocellulosic biomass and triglycerides from vegetable oils. The development of ecologically friendly and sustainable energy sources is aided by using supercritical fluids in the bio-fuel production process [20].
- Carbon Capture and Storage: Supercritical fluids, especially supercritical CO2, have gained attention in the field of carbon capture and storage (CCS) due to their ability to efficiently capture and transport CO2. In CCS processes, supercritical CO2 is used to capture CO2 emissions from industrial sources, such as power plants or refineries. The high solubility of CO2 in supercritical fluids allows for effective separation and capture of CO2 [39]. After capture, the supercritical CO2 can be transported and stored in underground geological formations, such as depleted oil and gas reservoirs or deep saline aquifers. Supercritical fluids facilitate the safe and efficient storage of CO2, contributing to efforts to mitigate greenhouse gas emissions and combat climate change.
- Enhanced Oil Recovery: Supercritical fluids, particularly CO2, offer advantages in enhanced oil recovery (EOR) techniques. In EOR, supercritical CO2 is injected into oil reservoirs to mobilize and extract additional oil that is not easily recoverable using conventional methods [27].
Supercritical CO2 acts as a solvent and a displacing agent, reducing the oil viscosity and improving its flow properties. The high solubility of CO2 in oil allows for effective extraction and enhanced oil production. In addition, supercritical CO2 can also help in the extraction of residual oil trapped in reservoirs after primary and secondary recovery methods have been exhausted. EOR techniques using supercritical fluids contribute to increased oil production and improved resource utilization [21].
Supercritical fluids in pharmaceutical industry
Supercritical fluids have gained significant attention in the pharmaceutical industry due to their unique properties and versatile applications. They provide valuable tools for the formation of new drug delivery methods, encapsulation, solubility augmentation, and drug formulation. Supercritical fluid technologies are a viable avenue for pharmaceutical research and development because they offer exact control over particle size, enhanced drug solubility, and the capacity to construct novel drug delivery systems [22].
- Drug Formulation and Encapsulation: Supercritical fluids, particularly supercritical carbon dioxide (scCO2), have been widely used for drug formulation and encapsulation. Supercritical fluid technologies offer a solvent-free and mild processing approach, which is crucial for the preservation of drug stability and bioactivity. Using techniques such as supercritical fluid extraction of emulsions (SFEE) or supercritical fluid technology-based encapsulation (SFTE), drugs can be encapsulated within biocompatible materials, such as polymers or lipids, forming drug-loaded particles or microcapsules. Supercritical fluid technologies enable precise control over particle size, morphology, and drug release properties, leading to improved drug delivery and therapeutic efficacy [23].
- Supercritical Anti-Solvent Precipitation: Supercritical anti-solvent (SAS) precipitation is a technique widely employed for the production of drug particles with controlled size and morphology. In SAS precipitation, a drug solution is rapidly mixed with a supercritical fluid anti-solvent, causing the drug to precipitate out as fine particles. This method has advantages over conventional precipitation techniques, including higher drug dissolving rates, less agglomeration, and better control over particle size. Particle size and drug properties can be accurately controlled by varying the process parameters, such as temperature, pressure, solvent choice, and mixing conditions. Drugs can now be produced in nano-scale particles with greater bioavailability and therapeutic efficacy because to the successful application of the SAS precipitation [24].
- Solubility Enhancement and Drug Delivery Systems: Supercritical fluids, particularly scCO2, have been utilized to enhance the solubility of poorly soluble drugs. The unique properties of scCO2, including its low viscosity, high diffusivity, and tunable density, allow for efficient drug solubility. Techniques such as supercritical fluid-assisted spray drying (SASD) or supercritical fluid technology-based particle coating can be employed to enhance drug solubility and create drug delivery systems with improved bioavailability. Supercritical fluid technologies enable the production of drug-loaded particles, micro particles, or solid dispersions, which can be formulated into various dosage forms, including capsules, tablets, or inhalation formulations [25].
Supercritical fluid as a refrigerant
A refrigerant is a substance or mixture, usually a fluid, used in a heat pump and refrigeration cycle. In most cycles it undergoes phase
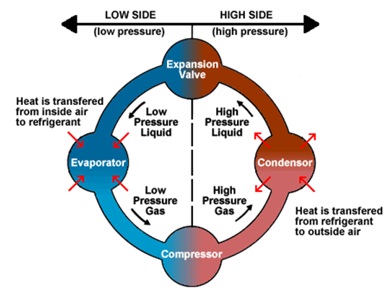
Figure 2: Refrigeration cycle [16].
transitions from a liquid to a gas and back again. Fluorocarbons, especially chlorofluorocarbons, became commonplace in the 20th century, but they are being phased out because of their ozone depletion effects [26].
The ideal refrigerant would be safe, free from toxicity and flammability, and have favourable thermodynamic properties. It would also not corrode mechanical components. When compared to other refrigerants, carbon dioxide has exceptional thermo-physical and transport properties [27]. The refrigerant is beneficial to the environment. It is compatible with polyester oil, non-flammable, and chemically inert. At the saturation temperature of CO2, a low ratio of vapour density to liquid density results in a high momentum for the vapour phase, better shear force between the liquid and vapour flow, and homogeneous flow in tiny channels. That is a basic reason, carbon dioxide has heat transfer coefficient 60 to 70% more than other refrigerants. The high vapour density also increases the heat absorbing capacity per unit volume flow rate of the refrigerant [28].
Supercritical fluids in green chemistry
Supercritical fluids have emerged as a powerful tool in the field of green chemistry due to their sustainable and environmentally friendly nature. They offer opportunities for solvent replacement, waste reduction, and the development of sustainable processes.
Supercritical fluids, such as supercritical water and carbon dioxide, have found applications in various green chemistry processes, including supercritical water oxidation and carbon dioxide utilization. Supercritical fluids provide sustainable alternatives to traditional solvents in chemical processes [29]. They possess unique properties that make them attractive from an environmental perspective. For example, supercritical carbon dioxide (scCO2) is non-toxic, non-flammable, and readily available, making it an excellent substitute for organic solvents. Supercritical water is another environmentally friendly medium that is abundant, non-toxic, and possesses unique properties for chemical transformations. Using supercritical fluids, chemical processes can be designed to minimize the use of hazardous substances, reduce waste generation, and enhance process efficiency [30].
One of the key advantages of supercritical fluids in green chemistry is their ability to replace conventional organic solvents. Organic solvents often pose risks to human health and the environment due to their toxicity and high volatility. Supercritical fluids provide an alternative solvent system that can enhance the sustainability of chemical processes. Using supercritical fluids as solvents, the need for large amounts of organic solvents can be significantly reduced or eliminated, leading to reduced environmental impact and waste generation. Furthermore, the unique properties of supercritical fluids, such as high diffusivity and low viscosity, enable efficient mass transfer and reaction rates, resulting in higher process efficiency and reduced reaction times Examples include Supercritical Water Oxidation and Carbon Dioxide Utilization [31].
Supercritical drying
Drying is a mass transfer technique that involves evaporating water or another solvent from a solid, semi-solid, or liquid. Before products are packaged or sold, this procedure is frequently employed as the last stage of production. A final product must be solid, such as a continuous sheet (like paper), long pieces (like wood), particles (like cereal grains or corn
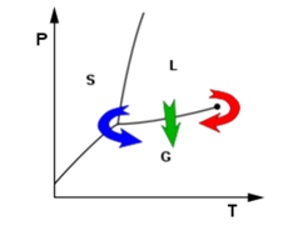
Figure 3: P-T Curve of Drying .
flakes), or powder (like sand, salt, washing powder, or milk powder) to be classified as "dried". There are frequently two components involved in this process: a heat source and an agent to remove the vapour that is created [12].
A precise and regulated method of removing liquid is called supercritical drying. A liquid body's substance transitions from liquid to gas at a limited pace as it passes the gas-to-liquid barrier, while the liquid's volume declines. Surface tension in the liquid body pushes against any solid structures the liquid may be in touch with when this occurs in a heterogeneous environment. This surface tension tends to break apart delicate structures like cell walls, the dendrites in silica gel, and the microscopic machinery of electromechanical systems. Conversely, supercritical drying occurs on the high-temperature, high-pressure side of the line, to the right of the line [23].
This route from liquid to gas does not cross any boundary, instead passing through the supercritical region, where the densities of the liquid phase and vapour phase become equal at critical point of drying. In most such processes, acetone is first used to wash away all water, exploiting the complete miscibility of these two fluids. The acetone is then washed away with high pressure liquid carbon dioxide. The liquid carbon dioxide is then heated until its temperature goes beyond the critical point, at which time the pressure can be gradually released, allowing the gas to escape and leaving a dried product. Air (evaporative) drying of specimens can cause severe deformation and collapse of structure, the primary cause of such damage being the effects of surface tension. The specimen is subject to considerable forces, which are present at the phase boundary as the liquid evaporates [32].
Water, the most used specimen medium, has a surface tension that is higher than air; acetone has a surface tension that is significantly lower. By switching out the liquid for one with a lower surface tension, the surface tension could be lowered and the drying process's damage could be minimized. Without experiencing a dramatic change in state, it is feasible to transition from a liquid to a gas at this "critical point." To avoid the harmful effects of surface tension, if the specimen had been in the liquid during this process, it would have undergone a transition from a "wet" to a "dry" gas environment without coming into contact with a surface. This process is employed in the creation of aerogels [33].
Supercritical fluids in materials science
Supercritical fluids have gained significant attention in materials science due to their unique properties and versatile applications. They offer various advantages for particle formation, coating and deposition techniques, and have found applications in drug delivery systems, nanotechnology, and thin films [34].
- Particle Formation and Size Control: Supercritical fluids have been extensively used for the formation of particles with controlled size, shape, and morphology. The process of particle formation using supercritical fluids is known as supercritical fluid technology or supercritical fluid precipitation [27].
- Supercritical Anti-Solvent (SAS) Process: The SAS process involves dissolving a solute in a supercritical fluid and rapidly precipitating it by injecting it into an anti-solvent. As the supercritical fluid rapidly expands upon depressurization, the solute precipitates, resulting in the nanoparticles formation. The size and morphology of the particles can be controlled by adjusting process parameters such as pressure, temperature, and flow rates.
- Supercritical Fluid Crystallization (SFC): The process of supercritical fluid crystallization is used to create crystalline materials with precise particle sizes and crystal shapes. A solute is dissolved in a supercritical fluid and then allowed to cool or depressurize under controlled conditions in order to cause crystallization. The synthesis of specialized chemicals and medicinal molecules benefits greatly from the usage of this process [28].
- Supercritical Fluid Spray Drying: Supercritical fluid spray drying is a technique used to produce dry powders from liquid solutions or suspensions. The process involves atomizing a solution or suspension in a supercritical fluid, which rapidly evaporates, leaving behind fine particles. This method offers advantages such as enhanced control over particle size, improved product stability, and the ability to process heat-sensitive materials.
- Coating and Deposition Techniques: Supercritical fluids provide an effective medium for coating and deposition processes in materials science. They offer unique properties that enable precise control over film thickness, uniformity, and composition [35].
- Supercritical Fluid Deposition (SFD): Utilizing supercritical fluids as a carrier for precursor materials that are subsequently deposited onto a substrate is known as supercritical fluid deposition. Thin films with regulated morphology and content can be produced using this method. Supercritical fluids have low viscosity and high diffusivity, which facilitate effective precursor transport and film formation.
- Supercritical Fluid Assisted Atomization (SAA): The process of supercritical fluid aided atomization is used to create nanostructured coatings and particles. In a supercritical fluid, a solution or suspension containing the intended coating material is atomized. The supercritical fluid rapidly expands and evaporates, leaving behind nanostructured coatings or particles [36].
Conclusion
Supercritical fluids have demonstrated their versatility and power in a wide range of applications across multiple sectors. They are very beneficial for a variety of processes and operations because of their special qualities, which include density, compressibility, viscosity, solvating power, and transport capabilities. We have emphasized the significance of supercritical fluids in several domains through this thorough investigation of them and their applications. Starting with the properties of supercritical fluids, we have discussed how these properties contribute to their distinct behaviour, bridging the gap between gases and liquids. The ability to control these properties by manipulating pressure and temperature conditions has opened up numerous possibilities for practical applications. One of the prominent applications of supercritical fluids is in extraction processes. Supercritical fluid extraction offers a solvent-free and environmentally friendly alternative to conventional extraction techniques. It has been successfully employed in the extraction of natural products, essential oils, flavours, and fragrances. The superior selectivity, efficiency, and mild operating conditions of supercritical fluid extraction make it an attractive option in the food, pharmaceutical, and cosmetic industries.
The unique properties of supercritical fluids allow for improved resolution, shorter analysis times, and enhanced sample throughput. This technique finds applications in various areas, including pharmaceutical analysis, food analysis, and environmental analysis. Supercritical fluids also find extensive applications in materials science, particularly in particle formation, size control, coating, and deposition techniques. These applications have significant implications in drug delivery systems, nanotechnology, and thin films, enabling precise control over particle characteristics and surface properties. In the pharmaceutical industry, supercritical fluids offer unique opportunities in drug formulation and encapsulation, solubility enhancement, and the development of novel drug delivery systems.
Therapeutic efficacy and patient care are made possible by improvements in drug solubility, targeted drug delivery, and exact control over particle size. Various applications of the supercritical fluids have transformed a number of sectors. Innovative procedures and technologies have been made possible by their remarkable qualities and controllable attributes. The potential of supercritical fluids has not yet been completely realized, indicating a future of ongoing innovation and application development as this field of study advances.
Acknowledgments
The authors would like to appreciate the Central Laboratory Federal University Wukari, Taraba State.
Conflict of interest
The authors declare that there is no conflict of interest in this article.
Orcid
Humphrey S. Samuel : 0009-0001-7480-4234
Emmanuel Edet Etim : 0000-0001-8304-9771

